Although the residual moisture and the glass transition temperature (Tg) can be regarded as giving some indication as to the real time stability of the product, there is no substitute for real time stability studies. It is imperative that real time stability studies are carried out with lyophilized LBPs as early as possible, since this data will help to support a clinical trial application (IMPD, IND). While accelerated stability studies can help to predict the real time stability, artefacts can occur if the glass transition temperature (Tg) is relatively close to the accelarated temperature, i.e. close to the onset of the Tg. Therefore, BioLyo offers stability studies that can be executed at -80, -20, +5 and +25 °C. Typically a condition like -80 or -20 °C is chosen to represent the temperature where no loss of viability takes place as a comparator, while +4 °C is generally chosen as target storage temperature to determine the shelf life. Room temperature such as +25 °C only makes sense to include as an accelerated temperature if the Tg is at least 35°C or higher.]
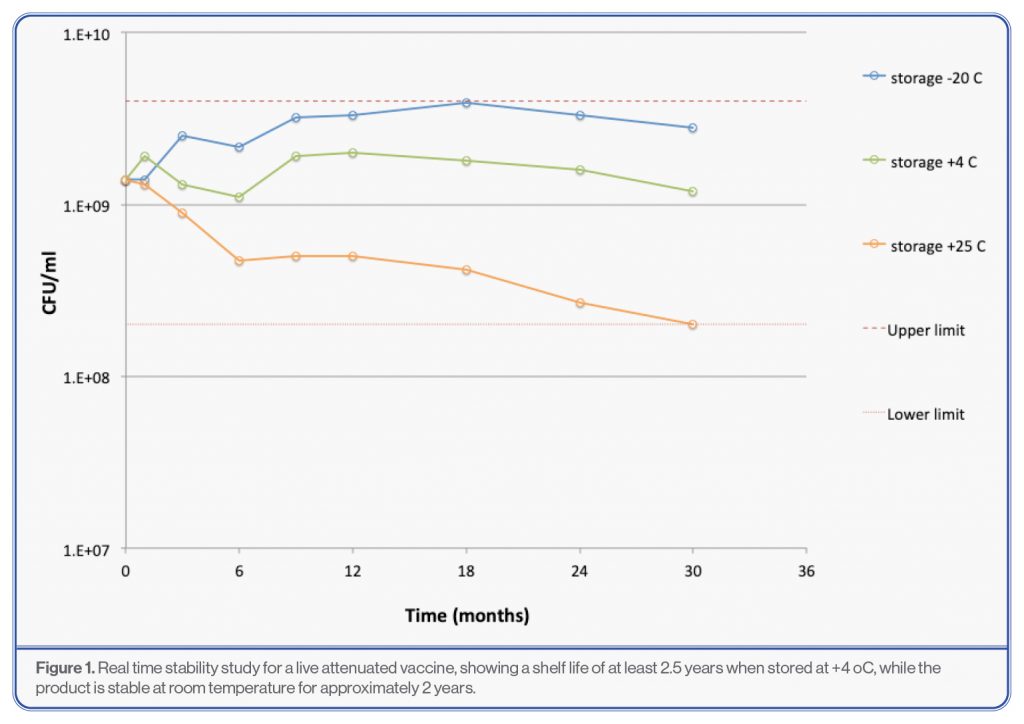
There is a misconception that a lyophilized product does not need refrigerated storage. While products may be stable at room temperature, there will always be certain limits within which the product will need to be stored, for example 18 – 25°C. If these limits cannot be guaranteed, it is safer to store the product in a refrigerator. For example, room temperature in India may vary from 18 to 36°C, while in Finland room temperature will seldom exceed 25°C. Therefore, storage at room temperature in Finland is fine while for India it is safer to store the product in a refrigerator.