The attrition rate of biotech products that are clinically evaluated in a phase I study is quite high. Therefore the willingness to develop a process that is robust and well defined is generally minimal prior to phase I GMP manufacture. However, it should be noted that for live biologicals the condition of the bacterial cells during release into for example the GI tract, can have a significant impact on whether or not the organisms colonize or not. Thus, a phase I trial may fail due to a poorly manufactured product, not necessarily because the treatment itself cannot work. It is understood that there is has to be a balance between process development time needed and the quality of the process & product, but only too often the absolute minimum is spent on process development prior to GMP manufacture of a product for phase I. In other words, the LBP that is being evaluated in the clinic should be manufactured according to a robust and repeatable process so that the resulting Drug Product has the best possible chance to demonstrate a clinical effect.
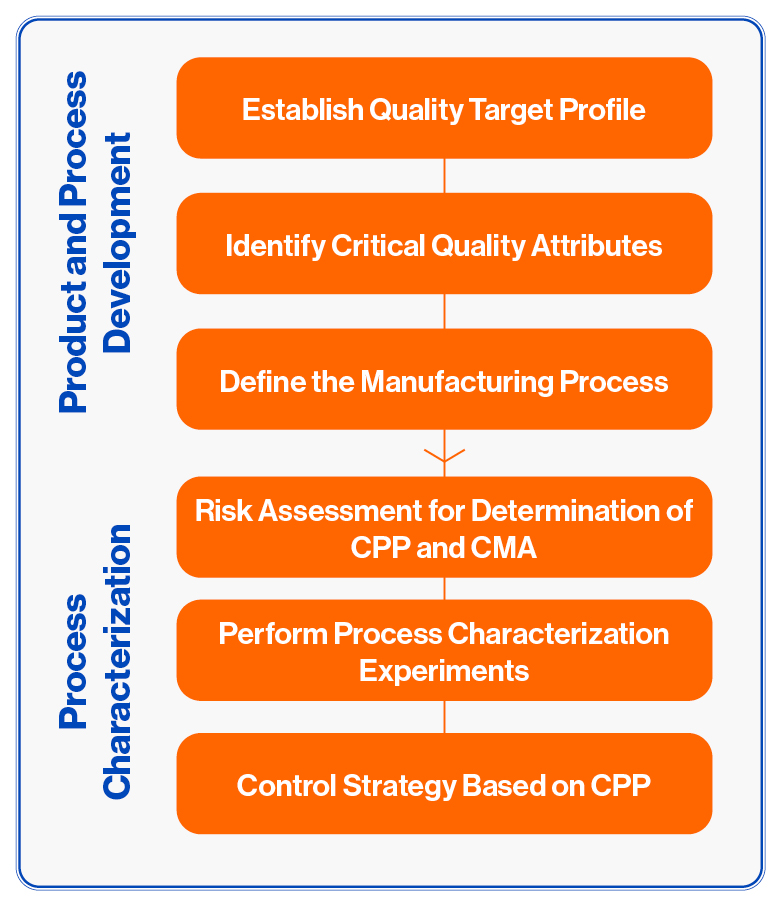
Once the phase I and II studies yields results that further warrant the clinical evaluation of the product, the production process needs to be characterized at small scale prior to process validation for phase III clinical trials. Currently there are very few CDMOs that provide process characterization services, let alone for LBPs. Typically the small scale process characterization is initiated once the decision has been made to start planning the phase III clinical trials.
The objective of process characterization is to determine the Critical Quality Attributes (CQAs) of the Drug Product, and the impact that the Critical Process Parameters (CPPs) have on the CQAs. Process characterization is a fair amount of work, but will lead to the determination of a Design Space, i.e. a set of process parameters that always leads to a Drug Product that passes all QC testing criteria. During Process Validation, the CQAs and CPPs are confirmed at the commercial manufacturing scale. Both the process characterization and process validation in combination with the stability data are the most important parts of the CMC section of a BLA submission.